Newborn (Genetic) Screening
Genetic Test
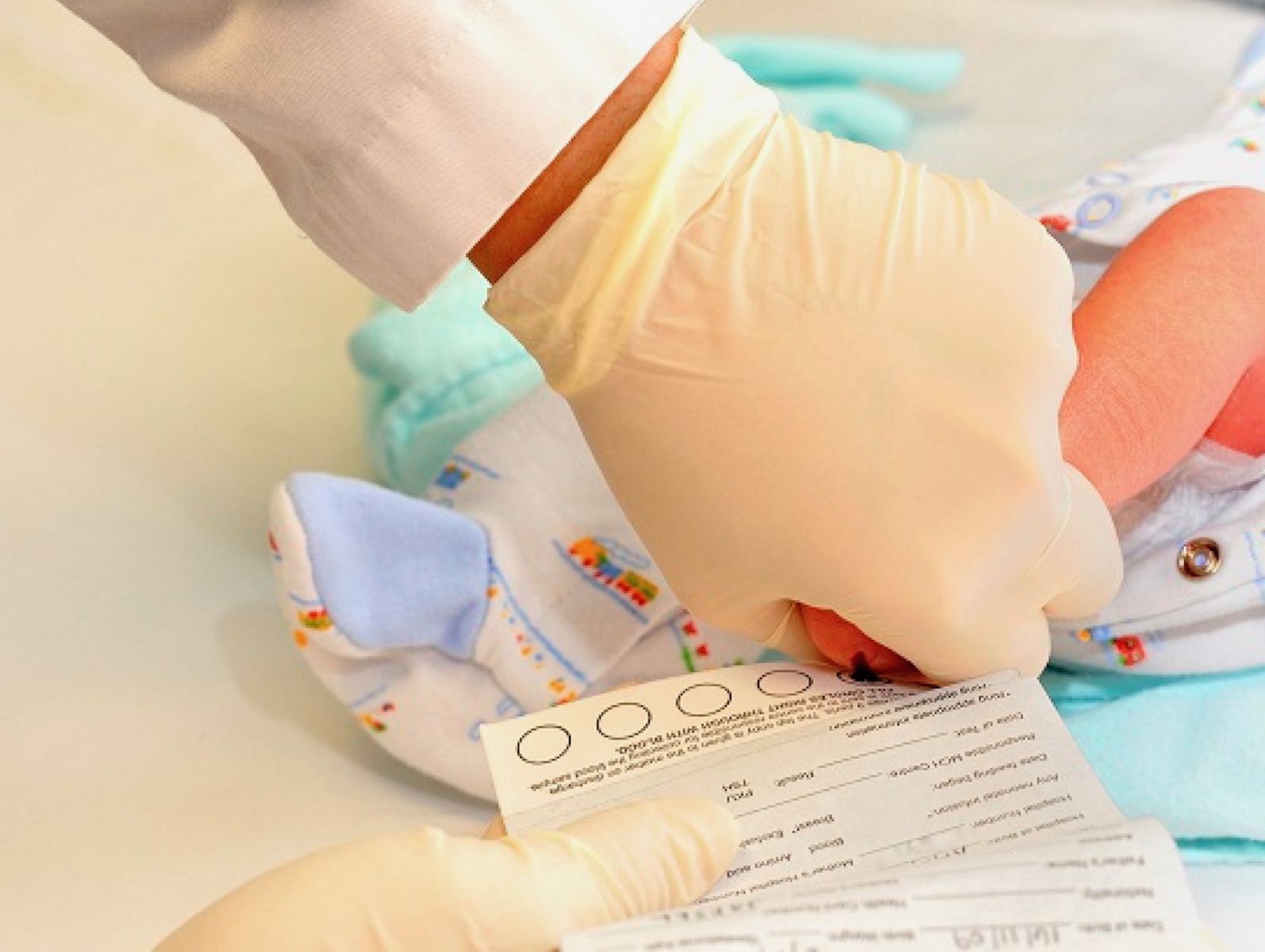
Every state has a mandatory newborn screening program to test for serious genetic disorders and genetic traits in newborns.
Within 48 hours after the birth of a baby, the heel is pricked, newborn blood is squeezed onto a card with special filter paper, and the card is sent for analysis to the State government laboratory or a laboratory under contract with the state Department of Health. At least 4.0 million newborn babies are tested every year in the United States.
Parents are typically not asked for permission before the newborn genetic testing is done. Most states mandate the testing. No consent is required. Some states allow parents to opt out of testing, particularly for religious reasons. However, parents are often not informed that opting out is possible. In fact, most parents have no idea that testing is even being done.
However, if given a choice, most parents would likely choose to have their child tested to speed up the diagnosis and treatment of these relatively rare newborn disorders. Read more >>>
Storage of Newborn DNA
After the newborn screening is complete, state health departments save and store the residual dried blood spots (DBS), some for only 3 months, some indefinitely.
At least 16 states store newborn dried blood spots for 10 years or longer. The Newborn Screening Saves Lives Act, reauthorized every 5 years, may encourage all states to hold on to "Baby DNA" long-term.
Parents are also not told about the storage of their baby's blood spots (DNA). This nationwide collection of newborn DNA has been called a "national treasure." Read more >>>
Find out how long your state stores newborn DNA.
As a result of 5 parent lawsuits (IN, MI, MN, TX), many states let parents opt out of storage. Contact your state health department or look for an opt-out form.
Research on Newborn DNA
State Health Departments conduct research and share newborn DNA with private and government researchers. Research using these dried blood spots includes, but is not limited to, the development of additional newborn screening tests (test development), research into newborn and adult-onset medical conditions, the impact of environmental pollutants, and analysis of genetic variation in the human population.
In 2002, federal officials met in North Carolina to discuss the creation of a centralized national biobank of newborn dried blood spots. There have been discussions of creating a virtual dried blood spots biobank with networked access to state repositories of banked newborn DNA. Read more >>>
Research is typically conducted without parent consent.
As a result of five parent lawsuits (IN, MI, MN, TX), many states let parents opt out of research. Contact your state health department or look for an opt-out form.
Comprehensive Overview
Read a 10-point overview or for more information, go to www.itsmydna.org





